Your Professional Team for
Regulatory Affairs

Efficient – Pragmatic – Helpful
We have Everything Important on the Radar
Whether for a project or as your external RA department. We manage your admissions. And more. We love and live Regulatory Affairs.
With the experience of hundreds of different approvals with a wide variety of challenges, our team of complementary experts, and good contacts to all relevant authorities, your Regulatory Affairs tasks are in the best hands with us.
We provide you with tailor-made and reliable support
- For individual approval projects
- For a limited period of time (e. g. as a maternity leave replacement)
- Permanent as your external RA department
You can also rely on our Quality Check at any time thanks to the 4-eye principle. And thanks to back-ups, your admission projects are always “work in progress” with us.
Team Regulatory Affairs
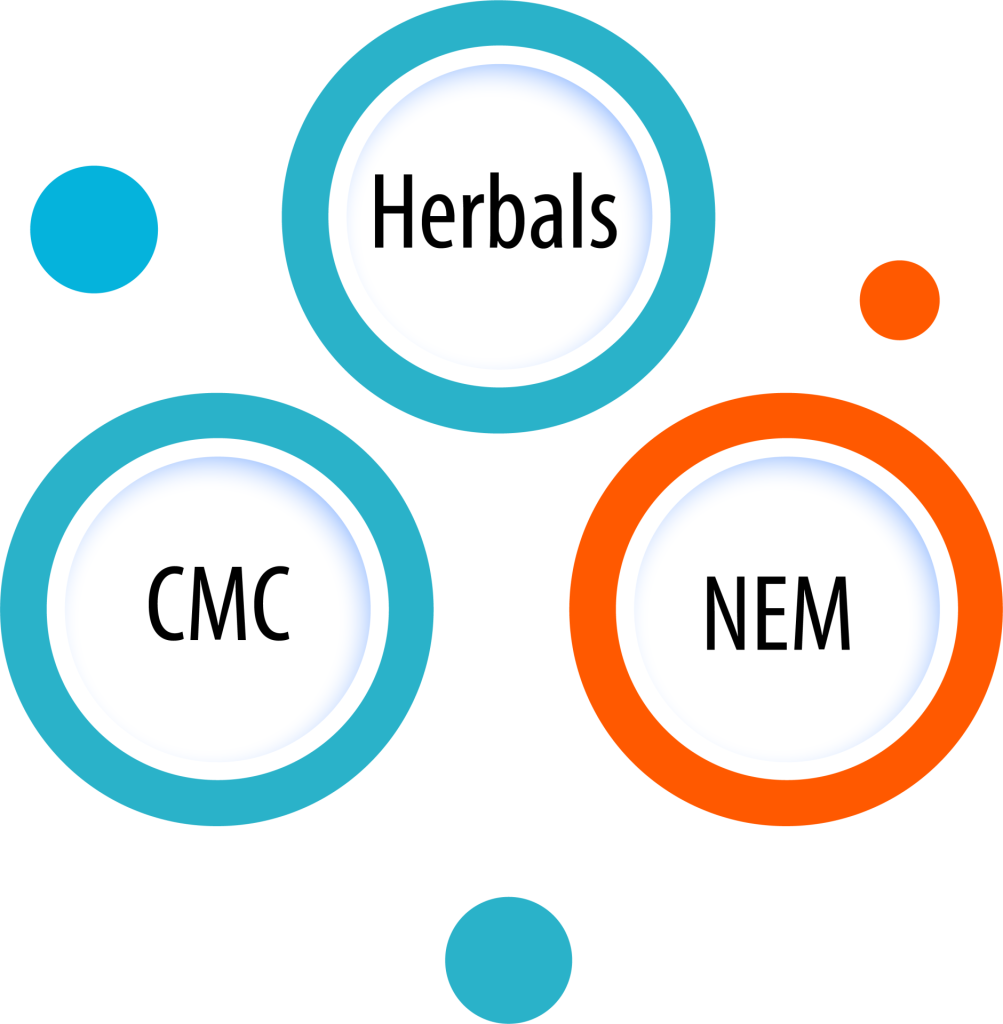
Our Key Areas
Our Special Areas
Our team of experts has experience with all types of approvals and accompanies your projects at all stages. Over the years, three areas have emerged in which we have been able to develop special expertise thanks to personal interests and numerous completed projects:
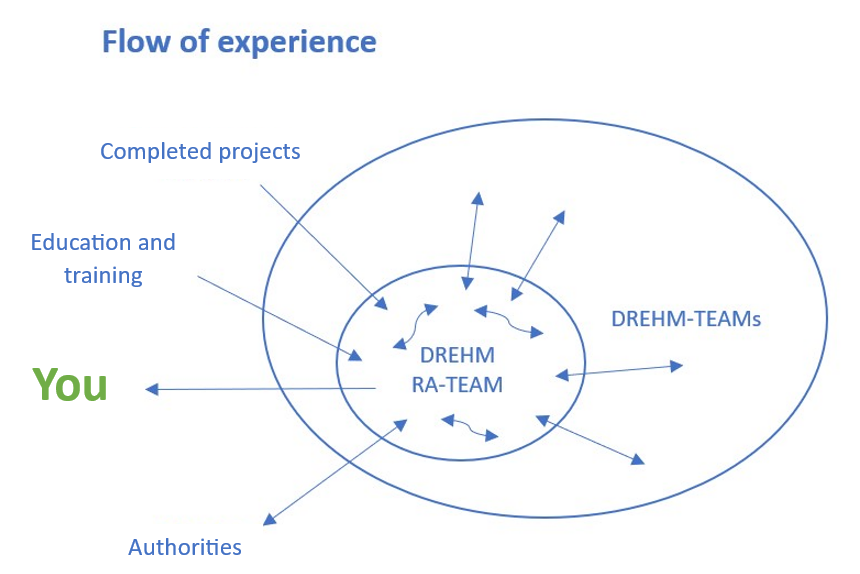
You learn with Us
“Sharing The Experience” is the motto of DREHM. And you, as our customer, benefit in particular: be it through the ongoing exchange with our specialists, by answering your technical questions, or by providing tailor-made training for you and your team.